electron configuration radon|full electron configuration of platinum : Tagatay The arrangement of electrons in radon in specific rules in different orbits and orbitals is called the electron configuration of radon. The electron configuration of radon is [ Xe ] 4f 14 5d 10 6s 2 6p 6 , if the electron arrangement is through orbitals. MMTCI RACE REWIND RESULTS AND DIVIDENDS OF BATANG PISTA MAY 31, 2023 WEDNESDAY RACE REWINDHere are the results and dividends of the races at Metro Manila Tur.
PH0 · where does radon gas come from
PH1 · unabbreviated electron configuration for barium
PH2 · radon nikodym theorem
PH3 · is radon really a problem
PH4 · interesting facts about radon
PH5 · full electron configuration of platinum
PH6 · electron configuration of all elements
PH7 · electron configuration chart
PH8 · Iba pa
12 talking about this. DISCLAIMER: FAN PAGE ONLY | UNOFFICIAL PAGE.
electron configuration radon*******The arrangement of electrons in radon in specific rules in different orbits and orbitals is called the electron configuration of radon. The electron configuration of radon is [ Xe ] 4f 14 5d 10 6s 2 6p 6 , if the electron arrangement is through orbitals.
A step-by-step description of how to write the electron configuration for Radon (Rn). In order to write the Rn electron configuration we first need to know the number of .
Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs. .
Radon electron configuration. ← Electronic configurations of elements. Rn (Radon) is an element with position number 86 in the periodic table. Located in the VI period. Melting .Radon is a colorless, odorless, and tasteless gas and therefore is not detectable by human senses alone. At standard temperature and pressure, it forms a monatomic gas with a density of 9.73 kg/m , about 8 times the density of the Earth's atmosphere at sea level, 1.217 kg/m . It is one of the densest gases at room temperature (a few are denser, e.g. CF3(CF2)2CF3 and WF6) and is the . Let us cover the interesting facts about electron density and distribution of electrons in Radon. How to write Radon Electron Configuration? The atomic number of .
Characteristics: Radon is one of the noble gases; hence it is a chemically inert, monatomic gas. It is also radioactive, colorless and odorless. Radon is produced naturally by the decay of uranium’s decay products, such as .
Radon is a chemical element of the periodic table with chemical symbol Rn and atomic number 86 with an atomic weight of 222 u and is classed as noble gas and is part of .
Electron Configuration. [Xe] 4f 14 5d 10 6s 2 6p 6. Rn. Upon condensation, radon glows because of the intense radiation it produces. Physical Properties. What is The Electron Configuration of Radon? Xe 4f14 5d10 6s2 6p6 is the electron configuration of the radon. How Many Valence Electrons Does Radon Have. Radon has eight valence electrons in its . Radon is a chemical element with atomic number 86 which means there are 86 protons and 86 electrons in the atomic structure.The chemical symbol for Radon is Rn. Electron Configuration and Oxidation States of Radon. Electron configuration of Radon is [Hg] 6p6. Possible oxidation states are 0. Electron Configuration Radon -. Rn: properties of free atoms. Radon atoms have 86 electrons and the shell structure is 2.8.18.32.18.8. The ground state electron configuration of ground state gaseous neutral radon is [ Xe ]. . Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full. . Electron configuration of Radon .
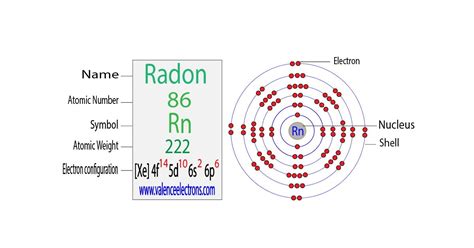
La configuration électronique du radon est Xe 4f14 5d10 6s2 6p6. Le radon est un élément chimique qui est représenté par le symbole Rn et dont le numéro atomique correspond à 86. Il en existe au total 37 isotopes, qui sont connus du 195Rn au 231R. C'est le plus lourd de ceux qui composent les gaz nobles, et chacun de ses isotopes a une .electron configuration radonThe electronic configuration of Radon is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6. What is the abbreviated electronic configuration of Radon? The abbreviated electronic configuration of Radon is [Xe] 4f14 5d10 6s2 6p6. To form abbreviated notation of electronic configuration, the completely filled subshells are .Answer: The electron configurations of the elements are presented in Figure 2.2.3, which lists the orbitals in the order in which they are filled. In several cases, the ground state electron configurations are different from those predicted by Figure 2.2.1. Some of these anomalies occur as the 3 d orbitals are filled.
Radon - Properties, history, name origin, facts, applications, isotopes, electronic configuation, crystal structure, hazards and more; Interactive periodic table of the chemical elements. . Electron Configuration [Xe] 4f 14 5d 10 6s 2 6p 6. Rn. Upon condensation, radon glows because of the intense radiation it produces. Physical Properties . What is The Electron Configuration of Radon? Xe 4f14 5d10 6s2 6p6 is the electron configuration of the radon. How Many Valence Electrons Does Radon Have. Radon has eight valence electrons in its outer shell. Radon Number of Valence Electrons.Électrons et configuration électronique. Le nombre d’électrons dans un atome électriquement neutre est le même que le nombre de protons dans le noyau. Par conséquent, le nombre d’électrons dans l’atome neutre de Radon est de 86. Chaque électron est influencé par les champs électriques produits par la charge nucléaire .

La configuración electrónica de Radon es Xe 4f14 5d10 6s2 6p6. El radón es un elemento químico que se representa con el símbolo Rn y cuyo número atómico corresponde a 86. Hay un total de 37 isótopos del mismo, que se conocen desde el 195Rn hasta el 231R. Es el más pesado de los que componen los gases nobles, y cada uno de sus isótopos .
Electron Configuration -The Electron Configuration of an Element Describes how Electrons are Distributed in their Atomic Orbitals. In Electronic Configuration electrons are arranged in various shells, .Elektronska konfiguracija radona. Elektronska konfiguracija radona je Xe 4f14 5d10 6s2 6p6. Radon je kemijski element koji je predstavljen simbolom Rn i čiji atomski broj odgovara 86. Postoji ukupno 37 njegovih izotopa, koji su poznati od 195Rn do 231R. Radon is a chemical element with the symbol Rn and atomic number 86. It is a radioactive, colorless, odorless, and tasteless gas that is the heaviest known gas and the sixth-heaviest known element. . Electron configuration. The electron configuration of an element describes the arrangement of electrons in the atoms of that element, and be .
ラドンの電子配置. ラドンの電子配置はXe4f14d5s10p6です。. ラドンは、記号Rnで表され、原子番号が2に対応する化学元素です。. ラドンには、6Rnから6Rまでの合計86の同位体があります。. それは希ガスガスを構成するものの中で最も重く、その同位体のそれぞれ .
Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table. The electron configuration for the first 10 elements. H . Radon -. Rn: properties of free atoms. Radon atoms have 86 electrons and the shell structure is 2.8.18.32.18.8. The ground state electron configuration of ground state gaseous neutral radon is [ Xe ]. 4f14. 5d10. 6s2. 6p6 and the term symbol is 1S0. Schematic electronic configuration of radon. The Kossel shell structure of radon.electron configuration radon full electron configuration of platinumIn this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83). The periodic table gives the following electron configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p65s2 4d10 5p6 6s2 4f14 5d10 6p3.
See the Greece Powerball Results 2022 here separated by month, including the draw history for 2022 of all draws. See if your lucky Greek Powerball numbers have won.
electron configuration radon|full electron configuration of platinum